Which Definition Best Describes the Term Molar Mass
The word mass in science means the density of an object. The molar mass of any substance can.
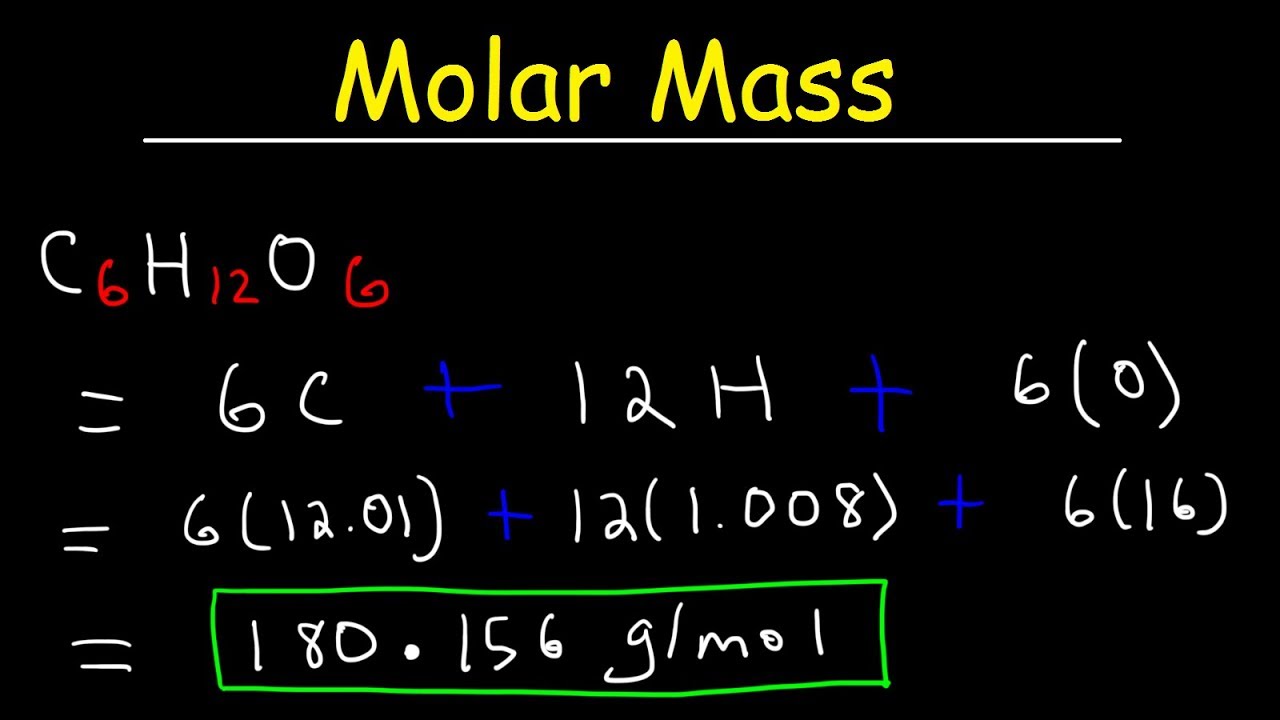
How To Calculate The Molar Mass Of A Compound Quick Easy Youtube
The molar mass of element A is 4 gmol.

. So to convert mole to mass rearrange the above equation mass in grams 1 mole x molar mass. Molar mass of 16 g. Molar mass is a term applied for molecules not for other objects.
To make you understand how molar mass and Molecular mass is different from each other here are the some of the major differences molar mass and molecular mass. Molar Mass of Elements. For example the atomic mass of an oxygen atom is 1600 amu.
The mass in g of 1 mole of a substance is known as the molar mass or molecular weight of the substance. Thus Catom 12 amu mol of. Now to prepare 05 M solution of FeCl₃.
Molar mass Mass of one particle N A Avogadros constant N A 6022 10 23 mol-1. Wāt 1. Molecular mass of a molecule is the mass of a mole of.
The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms. Element Molar Mass gmol silicon 2809 oxygen 1600. The molar mass of element B is 16 gmol.
This is because the mass is given per one mole. Molar Mass Molecular Weight - The term mole also referred to as mol was first used by Ostwald in 1896. Solved Which definition best describes the term molar mass.
Molar mass is defined as the mass in grams of one mole of a substance. The degree to which a body is drawn toward the earth by gravity. In statistics the process of assigning greater importance to some observations than to others or a mathematical factor used to apply such a process.
Molar masses of common chemicalcompounds that you might find in the chemistry laboratory can rangebetween. In other words it is the mass per mole of a substance. Generalizing this definition the molar mass of any substance in grams per mole is numerically equal to the mass of that substance expressed in atomic mass units.
Which definition best describes the term molar mass. The mass in grams of one mole of a substance Isotopes are atoms of a particular element that contain a different number of. Chemistry questions and answers.
Identify the molar masses of H and O. Difference Between Formula Mass and Molecular Mass Definition. What does the word mass mean in science term.
First we find molar mass and weigh it. Mass of 1 mole of oxygen is 159994 grams. The molar mass of a compound can be calculated by adding the standard atomic masses in gmol of the constituent atoms.
0 the mass in units of moles O the mass in grams of one mole of a substance O the mass in ounces of one mole of a substance O the mass of one gram of a substance. Mathematically Molar mass For example molar mass of 1 mole of molecule will be calculated as follows. The units for molar mass are in grams per mole.
See also Tables of Weights and Measures in the Appendix Abbreviated wt. Molar mass is the weight of one mole or 602 x 1023 moleculesof any chemical compounds. So 1 mole of any substance equal to its molar mass.
Calculate the molar mass of the compound NH 4 2 SO 4. 5g 1 mol1703 g. Defined as the sum o the atomic masses of all the atoms present in a molecule of a substance.
The molecular mass of a molecule is also called the molar mass. The molar mass is the mass of a given chemical element or chemical compound g divided by the amount of substance mol. Different elements have a unique molar mass value because they contain different numbers of electrons protons and neutrons.
The molar mass is the mass of a given chemical element or chemical compound g divided by the amount of substance mol. Which definition best describes the term molar mass. Therefore the molar mass 159994 gmol.
Formula mass is the sum of the masses of atoms present in the empirical formula. The molar mass of a given substance is defined as the mass of a sample divided by the moles of that substance in the sample. It is usually denoted by the symbol M.
Molar Mass is a physical property that represents the mass of a substance divided by the amount of that substance. When the initial mass of element A is 48 grams which mass of element B should be present. Multiply the molar mass with 05.
What does the term molar mass refer to. 16Use the table of molar masses to answer the question. Solution for 1 Determine the molar mass of MnClO42.
It is the mass in grams of one mole of a molecular compound. The molar mass also known as molecular weight is the sum of the total mass in grams of all the atoms that make up a mole of a particular molecule. The sum of the mass of the Mg2 ions and the mass of the Cl ions must be equal to the mass of MgCl2.
1 mole mass in grams molar mass. Calculate the molar mass of the compound NH42SO4. So 1 mole of FeCl₃ molar mass of FeCl₃.
The unit used to measure is grams per mole. Therefore 1 mole of molecules has a molar mass of 16 g. It can be used to find the number of molecules or atoms but not ions.

Molar Mass Molecular Mass The Molecular Mass Of A Substance Is The Mass In Atomic Mass Units Amu Of All The Atoms In A Given Molecule It Is More Commonly Ppt

Solved Which Definition Best Describes The Term Molar Mass Chegg Com

Molar Mass Molecular Mass The Molecular Mass Of A Substance Is The Mass In Atomic Mass Units Amu Of All The Atoms In A Given Molecule It Is More Commonly Ppt
No comments for "Which Definition Best Describes the Term Molar Mass"
Post a Comment